Both serological and Nucleic Acid Testing NAT is conducted to identify viruses. Our full range of donor testing including required supplemental and confirmatory testing is tailored to meet the regulatory and quality requirements of the industry.
 Low Sars Cov 2 Seroprevalence In Blood Donors In The Early Covid 19 Epidemic In The Netherlands Nature Communications
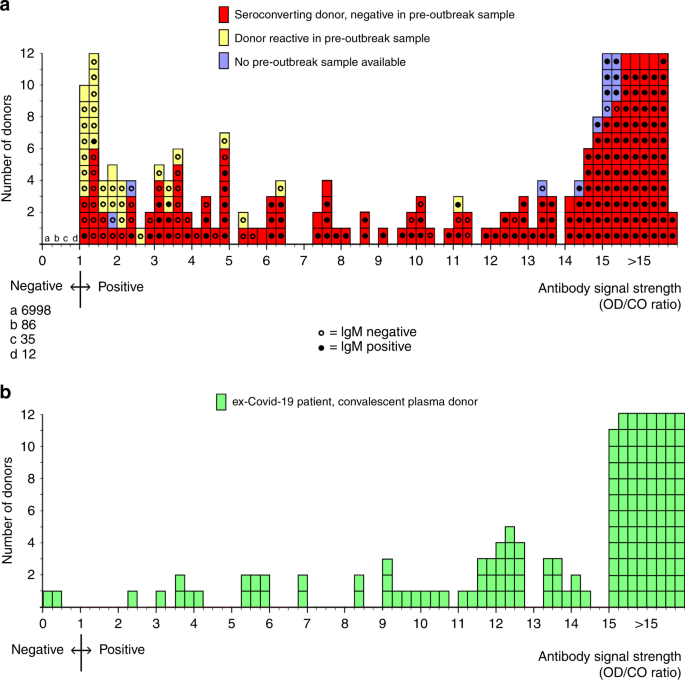
Low Sars Cov 2 Seroprevalence In Blood Donors In The Early Covid 19 Epidemic In The Netherlands Nature Communications
The Laboratories test millions of plasma samples each year ensuring the safety of the plasma.
Plasma donation testing. In order to lower the risk for TRALI in patients who are treated with CCP plasma for transfusion is usually collected from a person who has never been pregnant or a donor that tests. Plasma makes up 55 of human blood. The Department of Health and Social Care has asked NHS Blood and Transplant to stop collecting convalescent plasma donations.
After this diagnosis you can easily donate plasma. CoV-2 by a record of a validated diagnostic test at the time of illness. Donors should expect a physical examination and medical history review during their first plasma donation visit.
Plasma protein tests are blood tests that detect the amount of proteins in the blood. NAT testing allows certain viruses to be detected even before a donor displays any symptoms or develops antibodies. Additionally our test algorithm is designed to.
Besides the antibody test and COVID-negative tests haemoglobin HIV Hepatitis-B Hepatitis-C Syphilis and Malaria tests will be carried out before plasma donation. During each subsequent visit expect a review of your medical history and a check of your vital signs blood pressure pulse and temperature. Plasma is the clear straw-coloured liquid portion of blood that remains after red blood cells white blood cells platelets and other cellular components are removed.
All Grifols plasma centers are licensed and regulated by the FDA and other international agencies. 1 Blood Donor Educational Material 2 Donor History Questionnaires DHQ and Related Materials designed to assess both the safety of the donor and the blood collection 3 a focused health exam including hemoglobin screening 4 donor testing for transfusion-transmitted infectious diseases and 5 management of all donation. Donations from women who have been pregnant undergo additional testing for HLA antibodies to help prevent TRALI or transfusion-related acute lung injury.
Monograph Human plasma for fractionation. National Genetics Institute NGI offers comprehensive plasma donor screening for the global plasma industry and screens millions of plasma donations a year for blood-borne infectious agents. This follows the completed analysis of trial results which showed no overall benefit for people in hospital with coronavirus and a decision not to proceed with a third trial into plasma use early in the disease.
Administration of convalescent plasma is a treatment option for patients suffering from COVID-19 which has been tested world-wide. TRALI is a rare but serious complication that has been linked to transfusion of plasma. A negative lab test for active COVID-19 disease is not necessary to qualify for donation.
Donations will be tested for COVID-19 antibodies using samples obtained at the time of donation and sent to a laboratory where samples will undergo routine screening and infectious disease testing. Plasma is also different. Key elements of the blood donor screening process include.
B An interval of at least 14 days after full recovery. According to DonatingPlasma. It has been taken out of this guideline with the 4th revision and is.
Donor selection in. All blood platelet and plasma donations on or after June 15 2020 will also be tested for COVID-19 antibodies. This lab work is usually ordered as part of.
Corrigendum September 1999 included the scientific rationale for the deletion of the requirement for ALT testing. Each plasma donation center is inspected and certified by those agencies every two years in addition to being monitored by the International Quality Plasma Program IQPP. To the donation collection testing processing storage distribution and monitoring of convalescent plasma for the treatment of COVID-19 CCP.
Position paper on ALT testing CPMPBWP38599. Although further testing is required scientists believe plasma donated by patients who have recovered from the coronavirus could help those seriously ill from it Drugmakers are racing to develop a vaccine and treatment for the epidemic which has killed 1770 people and infected over 70500 people across China. This document is without prejudice to the requirements of the Union blood legislation any more stringent national measures in place at.
Annex VI Plasma-derived medicinal products. The requirement for ALT testing which has been deleted from the Ph. C Standard selection criteria for whole blood or plasma donation according to local requirements and standards age weight collection frequency vital signs freedom from deferral criteria in line with WHO Blood Regulators Network BRN.
Donors are asked questions about their history with hepatitis. In case of Covid the donation should only be done after 14 days of getting a negative report from a verified covid test. Individuals must have complete resolution of symptoms for at least 14 days prior to donation.
What are plasma protein tests. What are the tests carried out before plasma donation. A quick finger prick may be performed to test for anemia and your protein levels.
Since the levels of virus neutralising antibodies differ significantly in persons who have recovered from the disease reliable and simple tests are required which determine whether the plasma contains sufficient SARS-CoV-2 neutralising antibodies.
