But the FDA says theres no clock for that. But now that the Moderna and Pfizer vaccines have been distributed by the millions the drug companies have even more evidence to present to the FDA which they can.
 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
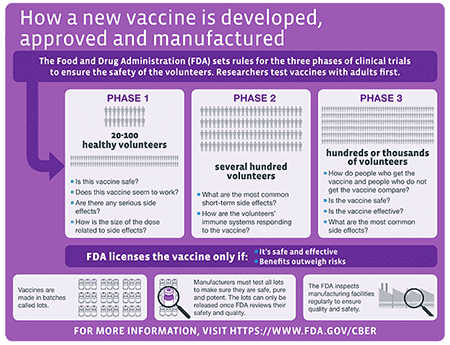
Currently the three Covid-19 vaccines distributed in the United States -- made by PfizerBioNTech Moderna and Johnson Johnson -- are authorized but not approved.

Are the vaccines fda approved. The FDA amended the emergency use authorization of the Johnson Johnson Janssen COVID-19 vaccine to include information about a very rare and serious type of. The FDA will review the results of these trials before approving COVID-19 vaccines for use. On Saturday the Food and Drug Administration FDA approved Johnson Johnsons Wuhan coronavirus vaccine making it the third coronavirus vaccine.
But because there is an urgent need for COVID-19 vaccines and the FDAs vaccine approval process can take months to years the FDA will first be giving emergency use authorization to COVID-19 vaccines based on less data than is normally. The FDAs approval today will add millions more vaccine doses to the national supply and brings hope that the country and the world can slowly make their way to recovery. Currently several COVID-19 vaccines are in clinical trials.
This EUA process is used in times of urgency to enable medications to become available more quickly than usual. The next step would be a Biologics License Application or a BLA. Even though the FDA granted emergency use authorizations for the PfizerBioNTech and Moderna vaccines in December 2020 the clinical trials the FDA will rely upon to.
Studies have not gone on long enough to evaluate long-term side effects. The road to FDA approval. It could also have an impact on vaccine mandates and perhaps sway skeptics hesitant to get the vaccines nowThe road to FDA approvalCurrently the three COVID-19 vaccines distributed in.
The manufacturers have to keep collecting data and continuing trials until they have enough information to submit for a BLA. Currently the three COVID-19 vaccines distributed in the United States made by PfizerBioNTech Moderna and Johnson Johnson are authorized but not approved. Vaccines of this type with respect to viruses are entirely new in humans.
Food and Drug Administration ushered in a new phase of the fight against COVID-19 on Friday by giving its blessing to a vaccine made. Modernas vaccine has been approved for use in people aged 18 and older. The currently available COVID-19 mRNA vaccines have been reviewed and authorized for use by the US FDA under an Emergency Use Authorization EUA process and so technically they are not considered fully approved.
Approval No COVID-19 vaccines have been approved. Earlier this week the FDA said Modernas vaccine was shown to be. Currently no coronavirus vaccine is fully approved by the FDA but three were given emergency use authorization by the agency Published April 14 2021.
The Pfizer and Moderna vaccines have emergency authorizations right now. Johnson Johnsons Janssen COVID-19 Vaccine. CDC and FDA have recommended a pause in the use of Johnson Johnsons JJJanssen COVID-19 Vaccine in the United States out of an abundance of caution effective Tuesday April 13.
90 rows April 23 2021. This page was updated on April 12 2021. Clinical trials are evaluating investigational COVID-19 vaccines in tens of thousands of study participants to generate the scientific data and other information needed by FDA to determine.
Approval means the FDA has officially decided that a product is safe and effective for its. This page was reviewed on March 24 2021.


