Sodium picosulfate DrugPatentWatch Estimated Key Patent Expiration Generic Entry Date. Food and Drug Administration today approved the first generic of Proventil HFA albuterol sulfate Metered Dose Inhaler 90 mcgInhalation for the treatment or prevention of.
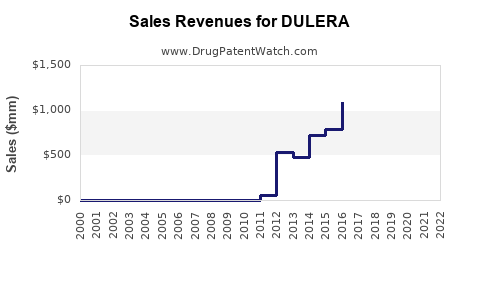 Dulera Market Exclusivity Period Mep When Will The Dulera Patents Expire And When Will Dulera Go Generic
Dulera Market Exclusivity Period Mep When Will The Dulera Patents Expire And When Will Dulera Go Generic
Infants born to mothers who have been using high doses of beclomethasone for.

When will dulera go generic. A generic therapy contains the same active components of an existing approved treatment. So as long as you have your doctors approval and so long as heshe writes the prescription you might be able to try this medicine. Posaconazole What it treats.
Tell your doctor about all the prescription and over-the-counter medications you use. Both medications are similar to Advair Symbicort Dulera and Breo. Is a drug marketed by Ferring Pharms Inc.
Mometasone furoate profile page. However lawsuits or other patents for specific uses of Dulera may delay the manufacturing of a generic version of the drug. And even if patients do not have to pay the full price they may still save money.
The generic ingredient in DULERA is formoterol fumarate. Mometasone Furoate What it treats. Asthma Expected savings per month.
Thats because many health plans have a lower co-payment for generics. DULERA should be taken every day as 2 puffs in the morning and 2 puffs in the evening. For adults and adolescents 12 years of age and older use DULERA 100 mcg5mcg or DULERA 200 mcg5mcg.
In fact 40 expensive brand drugs are expected to go generic in the next five years. Brand-only drugs like Restasis Eliquis and Lyrica can cost over 500 for a months supply and they dont have cheaper generic alternatives yet. Additional details are available on the formoterol fumarate.
It will be available in three different dose strengths of fluticasonesalmeterol. Qvar should be used only when prescribed during pregnancyThere are rare reports of harm to a fetus when the mother took other corticosteroids. Advairs generic treatment option has hit the market this truly is good news in that this product offers a 69-81 discount compared to the price of Advair.
A generic drug is an exact copy of the active drug in a brand-name medication. Dulera is available only as a brand-name medication. Dulera is a drug marketed by Merck Sharp Dohme and is included in one NDA.
It is expected to launch in the second half of February following label amendments required by the FDA. 100 mcg50 mcg 250 mcg50 mcg and 500 mcg50 mcg. Formoterol and mometasone for MOE ter ol and moe MET a sone Brand Name.
FDA drug approval initiatives will hopefully bring many generics to the market soon. A prescription medicine that may help control symptoms of asthma and prevent symptoms such as wheezing in people 5 years of age and older. In comparison this new generic inhaler costs between 50 and 90.
How to get it. Its not currently available in generic form. When the generic gets released.
Those typically cost between 300 and 400 per prescription. August 15 2021 Generic Entry Controlled by. Patents Listed in the FDA Orange Book Drug Database of DULERA-1 with information and expiryexpiration dates.
There are two patents protecting this drug and one Paragraph IV challenge. One supplier is listed for this compound. This includes vitamins minerals herbal products and drugs prescribed by other doctors.
240 When the generic gets released. There are nineteen drug master file entries for this compound. Mometasone furoate formoterol fumarate dihydrate inhalation.
If you miss a dose of DULERA skip your missed dose and take your next dose at your regular time. Last updated on March 1 2020. Medically reviewed by Philip Thornton DipPharm.
The earliest predictable date that a generic version of the drug could become available is July 2014 when the patent expires. DULERA combines an inhaled corticosteroid medicine ICS mometasone furoate and a long-acting beta 2.